Environmentally-friendly electrochemical regeneration of activated carbon
Opportunity
Activated carbon or charcoal is a specialised porous form of carbon. Its numerous pores increase the surface area available for molecules to adhere to—a process known as adsorption. Because of this, activated carbon is useful for applications involving filtration, purification, storage and extraction. In the environmental sector, activated carbon is used to purify air as well as remove dangerous and volatile compounds during wastewater treatment.
After use, adsorbed components within the activated carbon are removed or desorbed from the pores, allowing the activated carbon to be reused. This recycling process is called regeneration and can be performed via thermal, electrochemical, or microbiological methods among others.
Unfortunately, the high temperatures typically needed to desorb the adsorbed components means that regeneration is an energetically-costly process with a large carbon footprint. Many regeneration processes can only perform a limited number of regeneration cycles, with the efficiency of the reactivated carbon decreasing between cycles due to clogged or deteriorated pores.
Furthermore, most regeneration processes fail to address the proper disposal of the adsorbed compounds or the chemical by-products generated when removing these compounds, many of which are environmentally-toxic. These limitations reflect the need for novel regeneration methods that are more efficient and environmentally-friendly.
Technology
This invention describes an efficient and environmentally-friendly method for the adsorption and electrochemical regeneration of activated carbon in a single reactor. In this method, a constant stream of the compounds is fed into the reactor for adsorption by the activated carbon.
An electric current then generates hydroxyl radicals that desorb and degrade the compound, freeing the activated carbon for reuse using a methodology developed by the research team which does not involve additional equipment compared to the conventional method.
In doing so, this electrochemical method allows for multiple alternating cycles of adsorption and regeneration, with the activated carbon retaining up to 95% absorbency in between cycles. With the use of hydroxyl radicals, the method also overcomes the problem of having to dispose any toxic organic pollutants that were adsorbed. Because regeneration also occurs at the surface of the activated carbon, destruction of the material is limited—allowing its reuse and retaining efficiency.
Finally, the method can be performed at ambient temperature and pressure, reducing the energy requirement. Overall, the method’s environmental benefits, efficiency and ability to perform multiple adsorption/regeneration cycles makes it a cost-effective alternative to conventional regeneration methods.
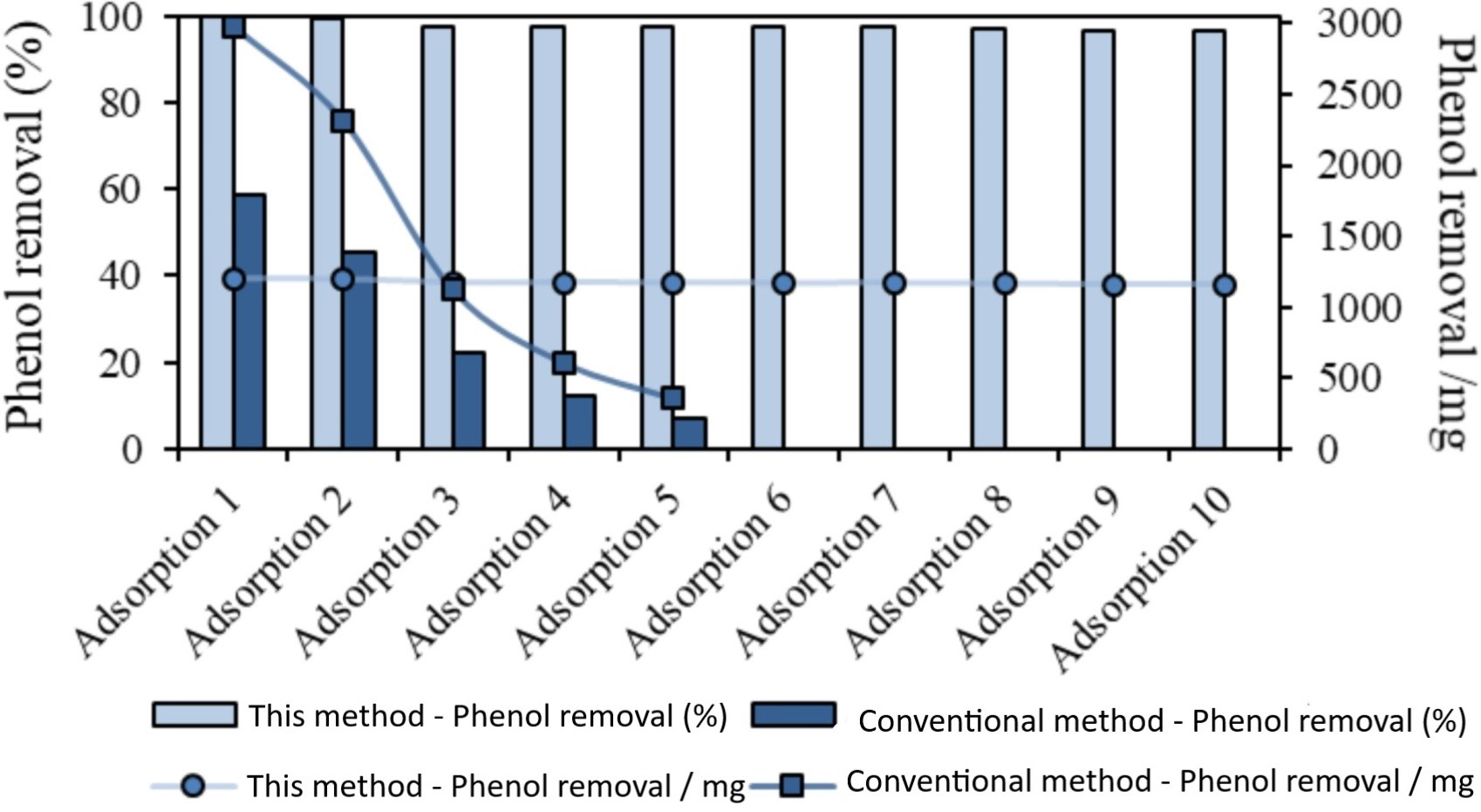
Comparison of adsorption and regeneration cycles using the method of this technology and the conventional method.


