Automating Rheumatoid Arthritis Assessment via Deep Learning
Opportunity
Rheumatoid arthritis (RA) is a chronic, autoimmune disease that most commonly afflicts joints in the hands, wrists and knees. It arises when the immune system mistakenly targets the body’s own tissues, triggering inflammation that could lead to irreversible damage in the long run. Patients with RA often complain of swelling, pain and stiffness of the joints. In the most severe cases, RA can cause limb deformity and, ultimately, loss of function.
There is no single test that can conclusively diagnose RA, and doctors typically have to run several to make sure. One such test is the X-ray, which helps visualise which joints are inflamed and to what degree. There are several methods for scoring X-ray radiographs, but most of them are manually graded, so physicians still need to attend to each image individually. As a result, interpretation of X-rays tends to be subjective and, depending on how well-staffed a hospital is, can take up to several days.
RA is the most common type of autoimmune arthritis. In Singapore, around one percent of the population have RA, equivalent to tens of thousands of radiographs requiring interpretation. There is therefore a need for an automated approach of processing these X-ray images quickly without sacrificing diagnostic quality.
Technology
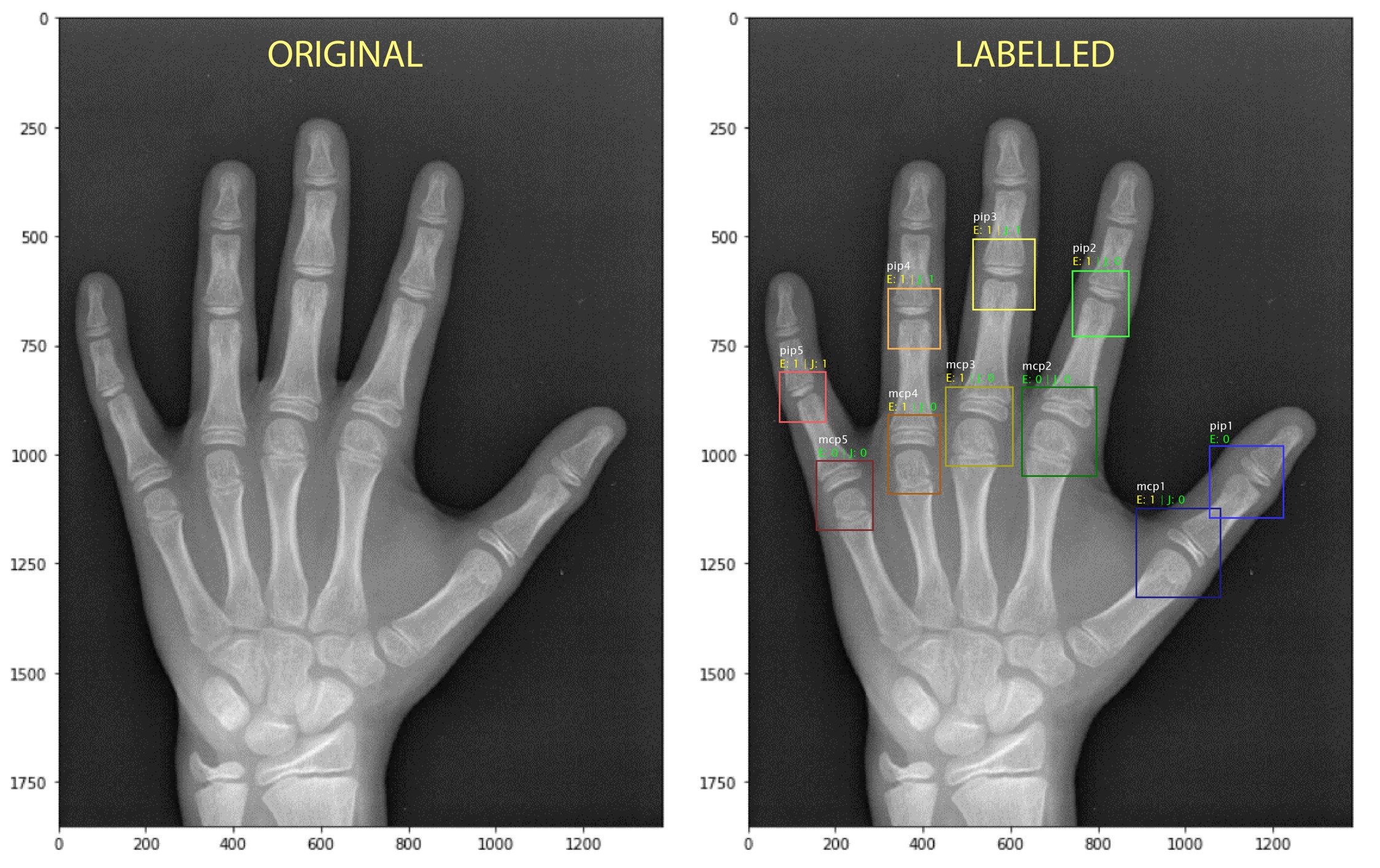
The described invention is a deep learning algorithm that automatically scores X-ray radiographs and accurately assesses the severity of joint damage. The novel tool starts by pre-processing the image to optimise its size, cropping and contrast, producing the best possible starting point for the algorithm. It then takes a joint segmentation approach and employs a variety of statistical, machine and deep learning techniques to train the prediction model. The result is a highly reliable tool that can detect the narrowing and erosion of hand joints with 97.30-percent and 94.63-percent accuracy, respectively. Accuracy rates for foot joints are similarly high.
As the technology does not rely on individual interpretations by different physicians, the model minimises subjectivity in RA diagnosis, and results can also be available within minutes. The tool also comes with a simple graphical user interface, making it usable even for technicians without extensive deep learning knowledge.
To achieve standards high enough for clinical use, the algorithm still needs improvements in terms of approach and accuracy. Training the predictive model on the local patient population, through partnerships with laboratories and hospitals, will also enhance its reliability and eventual clinical value.
Intelligent scoring of Rheumatoid Arthritis joints in minutes



