Metabolic Engineering for Efficient Lipoic Acid Production in Yeast
Opportunity
Found in our cells’ mitochondria, lipoic acid plays an essential role in aerobic metabolism by helping enzymes turn nutrients into energy. Notably, lipoic acid also has several health benefits. For instance, it can bind disease-causing free radicals, making it a promising antioxidant as a dietary supplement. Lipoic acid can also increase insulin sensitivity and has shown inhibitory effects against breast cancer cells, pointing towards its potential use against diabetes and cancer.
Traditionally, lipoic acid is chemically synthesised, requiring reagents and organic solvents that are toxic to human health and the environment. Moreover, current synthesis methods produce lipoic acid in two optically distinct forms, R and S, although only the former is known to have health benefits. To obtain pure R-lipoic acid, additional reagents and steps are needed, thus lengthening the production time, incurring higher manufacturing costs and leading to greater environmental impacts.
Biologically engineering microbial cell factories like baker’s yeast, a strain that is designated generally recognized as safe (GRAS), represents a promising avenue for obtaining pure R-lipoic acid in a sustainable and environmentally friendly manner. Unlike other microbes commonly used in biotechnology such as E. coli, baker’s yeast does not inherently consume free lipoic acid, hence potentially allowing its accumulation. Therefore, baker’s yeast is an attractive microbe for R-lipoic acid production and a method for engineering yeasts to elevate the amounts of R-lipoic acid produced is desirable.
Technology
This invention relates to a method of deploying metabolic engineering strategies in yeast to produce free R-lipoic acid. First, the presence of lipoic acid’s precursor and protein-bound lipase within baker’s yeast was first confirmed and characterised through liquid chromatography-tandem mass spectrometry (LC-MS/MS).
To liberate lipoic acid from protein-bound lipase found in the mitochondria, enzymes like lipoamidases are needed to break the amide bond between lipoic acid and the lipoylated proteins. Accordingly, a modified yeast strain expressing a bacterial lipoamidase in its mitochondria was developed, thus allowing for the production and accumulation of free lipoic acid.
Aside from lipoamidase, lipoic acid production can be further enhanced by overexpressing key biosynthesis enzymes like GCV3 and LIP2, as well as enzymes for regenerating cofactors like SAM2. By employing all these strategies, the production of lipoic acid in yeast was increased nearly three-fold. While more efforts are needed to further boost the yield, the method detailed demonstrates that metabolically engineering yeasts is a viable approach for lipoic acid production.
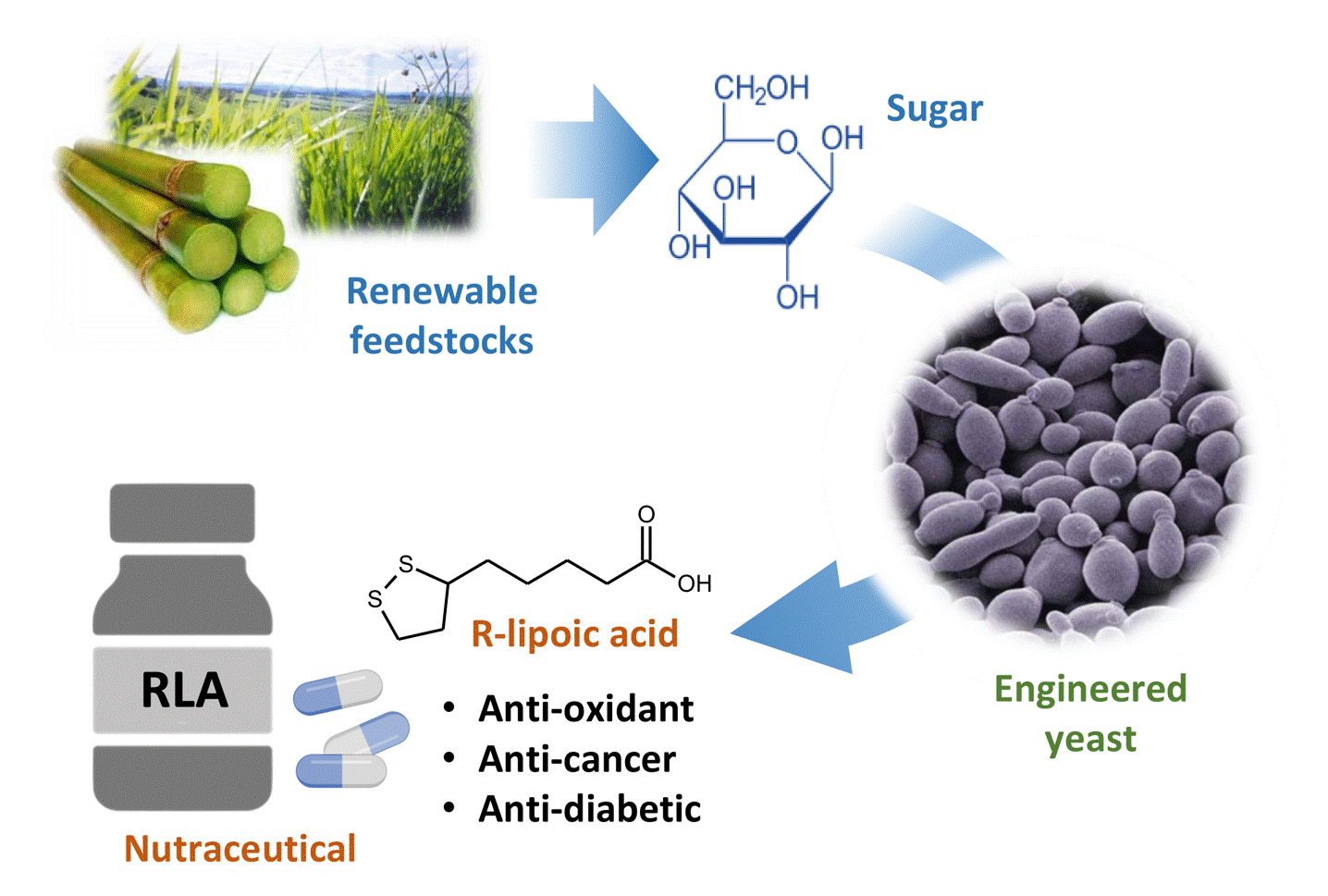
This technology uses an engineered yeast to convert sugar derived from renewable resources to pure R-lipoic acid, a valuable nutraceutical. Unlike chemical synthesis, this method requires neither harsh conditions nor toxic reagents, hence making it environmentally friendly and economical.


